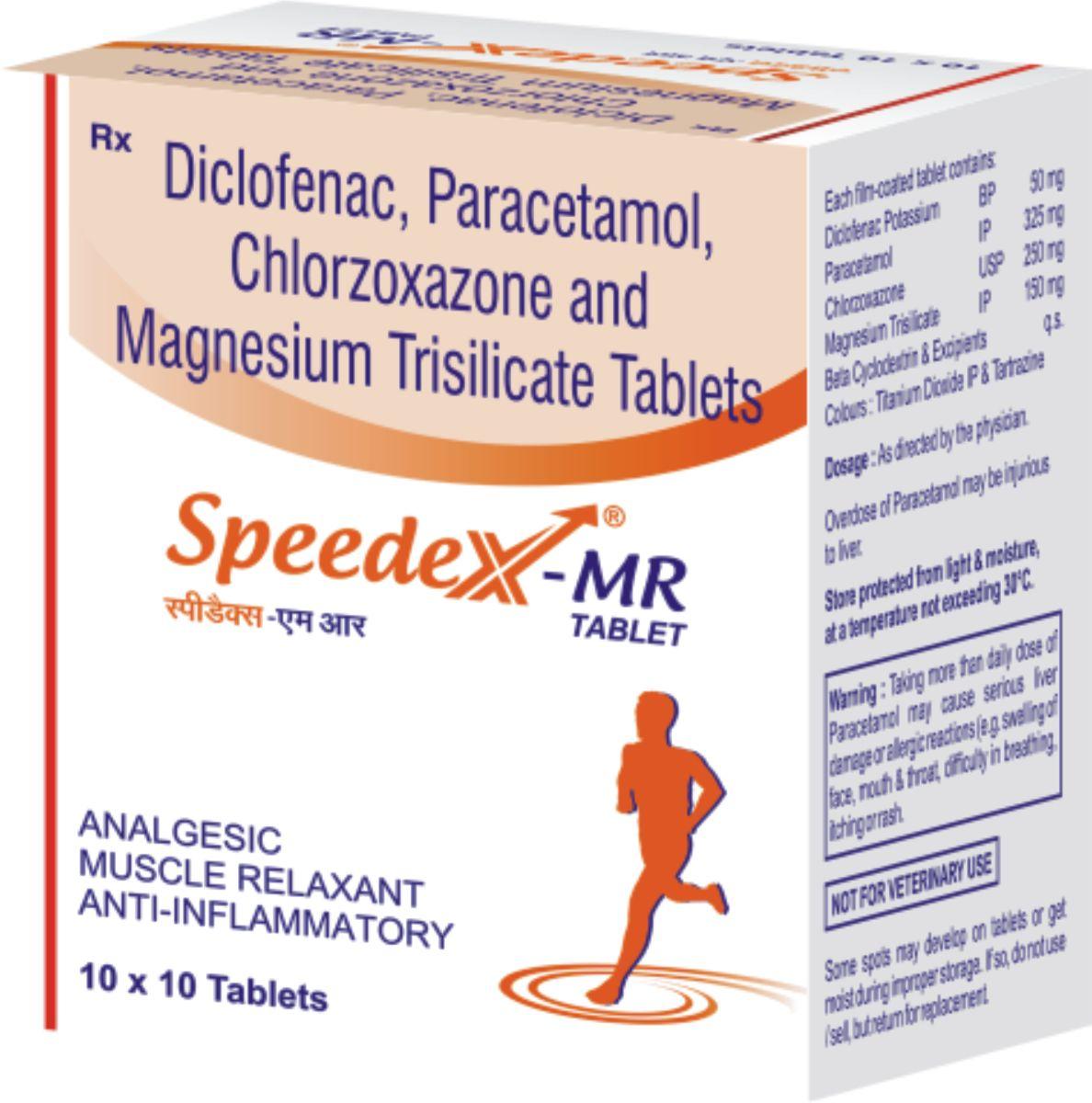
Speedex-MR
Description
| Composition: |
Each Speedex-MR tablet contains:
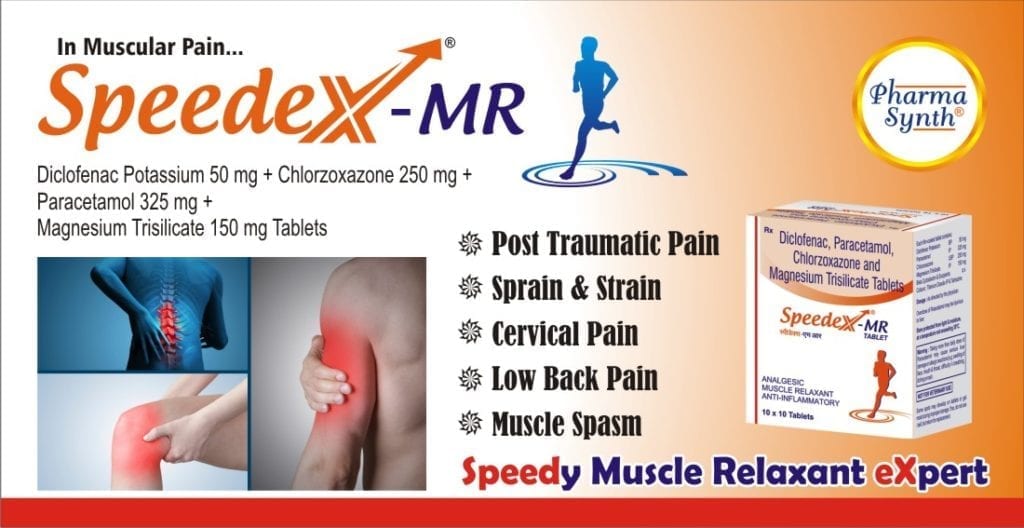
Paracetamol 325mg
Chloraxozone 250mg
Diclofenac potassium 50mg
Magnesium trisilicate 150 mg
| Dosage form: |
Tablets
| ATC classification: |
Analgesic and antipyretic
| Description: |
Speedex-MR is a modified release formulation complexing three analgesics and an antacid with β-cyclodextrins that provides high efficacy in pain relief with minimum side effects. With the β-cyclodextrins drug delivery in Speedex-MR it enhances bioavailability of the drugs and also reduces the irritancy of gastric mucosa that occurs with the use of analgesics. Paracetamol is the analgesic and antipyretic component. Chloraxozone is the skeletal muscle relaxant that blocks pain impulses that are sent to central nervous system (brain and spinal cord). Diclofenac is the non-steroidal anti-inflammatory drug present in Speedex-MR. With varied mechanisms of action these three analgesic acts immediately upon administration in relieving painful muscoskeletal conditions. Magnesium trisilicate counteracts the severe acidity that ensues due to the analgesics in the formulation.
| Pharmacological action: |
Paracetamol exerts analgesic effect by elevating pain threshold which is thought to be attained by inhibiting nitric oxide pathway mediated by a variety of neurotransmitter receptors including N-methyl-D aspartate (NMDA) and substance P. It shows antipyretic activity by inhibiting the synthesis and release of prostaglandin in the central nervous system, thereby inhibiting prostaglandin-mediated effects on the heat-regulating center in the anterior hypothalamus.
It is absorbed rapidly mainly from the small intestine producing peak plasma levels after 15-20 minutes following oral dosing. Its systemic bioavailability is dose-dependent and ranges from 70 to 90%. Food delays oral absorption. It is rapidly and widely distributed throughout the body with a volume of distribution of approximately 0.9L/kg and is eliminated from plasma with a half life of approximately 2 hours. It is extensively metabolised predominantly in the liver, the major metabolites being the sulphate and glucuronide conjugates which are excreted in urine.
Diclofenac works by inhibiting the enzyme, cyclooxygenase (COX), an early component of the arachidonic acid cascade that results in the formation of prostaglandins, thromboxanes and prostacyclin. Prostaglandins are produced at sites of injury or damage causing pain and inflammation. Diclofenac by blocking the effect of COX enzymes, reduce the prostaglandin formation, which means pain and inflammation are eased.
Chlorzoxazone inhibits multisynaptic reflex arcs in subcortical areas of brain and at the level of spinal cord resulting in reduction of skeletal muscle spasm with relief of pain and increased mobility of the involved muscles. Peak plasma levels, in the majority of the subjects, reach in about 1 to 2 hours after oral administration of chlorzoxazone. It is rapidly metabolized and is excreted in the urine, primarily in a conjugated form as the glucuronide.
The analgesics present in the Speedex-MR tablets are likely to cause gastrointestinal irritability and heartburn. Magnesium trisilicate formulated into Speedex-MR counteract these side effects by buffering hydrochloric acid in the stomach and increasing gastric pH.
| Indications: |
Speedex-MR is indicated for
- Severe muscoskeletal pain due to trauma or injury
- Sprain and strains
- Cervical pain
- Lower back pain or sprain
- Muscle spasms
- Pain and inflammation due to ankylosing spondylitis and spondyloarthritis
- Pain due to osteoarthritis, rheumatoid arthritis and gout attacks
- Pain and inflammation due to sports injuries
- Pain associated with cervical root and disc syndromes.
- Myalgias, torticollis and
| Dosage and Administration: |
Recommended dosage unless otherwise specified by Physician:
For adults: 1 Speedex-MR tablet two-three times in a day.
Dose may vary depending on severity of the condition.
| Contraindications: |
- Speedex-MR is contraindicated in patients with hypersensitivity or idiosyncrasy to any of its ingredients and NSAIDs
- Also in patients with GI bleeding, moderate to severe renal impairment and in third trimester of pregnancy.
- Diclofenac containing formulations should only be initiated after careful consideration for patients with significant risk factors for cardiovascular events (eg, hypertension, hyperlipidaemia, diabetes mellitus, and smoking).
| Side effects: |
- Speedex-MR is usually well tolerated. Report to your doctor if you observe any of the effects mentioned below.
- Occasionally patients may develop gastrointestinal disturbances.
- Patients may experience dizziness, nausea, lightheadedness, malaise, or over stimulation.
- Slight discoloration of urine
- Ringing or buzzing sound in the ears
- Stop the medication and seek immediate medical attention if the patient taking the medication experiences any severe reactions including a noticeable reduction in urination, weakness which affects the muscles, numbness, bloody stools and seizures.
| Warnings and Precautions: |
General:
- Inform your doctor if you are on any pain killers/NSAIDs.
- Drowsiness can occur with the use of Speedex-MR and may be additive to drowsiness from the concomitant use of alcohol or other central nervous system depressants.
- It is recommended to avoid use of machinery or driving when on this medication.
- Speedex-MR might increase the risk of serious cardiovascular events or intestinal bleeding. Ensure that you fully discuss the potential risks with your physician before beginning treatment.
- If you suffer from liver disease and gastrointestinal disorders, ensure that you make your physician aware of this before treatment begins, in order for your physician to determine whether or not this medicine is safe and suitable for you.
- Precaution to be exercised in patients with: Gastrointestinal bleeding, history of peptic ulceration, cerebrovascular bleeding, ulcerative colitis, Crohn’s disease, systematic lupus erythromatosus (SLE), porphyria, hematopoietic or coagulation disorders, Raynaud’s phenomenon.
Drug Interactions:
- The speed of absorption of paracetamol may be increased by metoclopramide or domperidone and absorption reduced by cholestyramine. The anticoagulant effect of warfarin and other coumarins may be enhanced by prolonged regular daily use of paracetamol with increased risk of bleeding; however occasional doses have no significant effect.
- Diclofenac may increase the plasma concentrations of lithium & digoxin. Concomitant use of diclofenac & diuretics may inhibit the activity of diuretics. The activity of anticoagulants may be enhanced when used concomitantly with diclofenac. It may increase toxicity of drugs like cyclosporine. NSAIDs may diminish anti-hypertensive action of ACE-inhibitors.
- Additive CNS depression may occur when chlorzoxazone is administered concomitantly with other CNS depressants, antipsychotics, antianxiety agents including alcohol.
Pregnancy and Lactation:
- This formulation should not be used in the third trimester of pregnancy.
- Not recommended even in first two trimesters of pregnancy and also during lactation period since safety in pregnant women or nursing mothers has not been established
| References: |
Diclofenac- https://pubchem.ncbi.nlm.nih.gov/compound/diclofenac#section=Drug-and-Medication-Information
Paracetamol-https://pubchem.ncbi.nlm.nih.gov/compound/acetaminophen#section=Top
Chloraxozone https://pubchem.ncbi.nlm.nih.gov/compound/chlorzoxazone
Cyclodextrins in delivery systems: Applications https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3147107/
Disclaimer:
Information provided above is for reference purpose only and has been compiled for use by healthcare practitioners. Please consult your physician to understand how the product affects you, its dosages, side-effects and further information.
Remember, keep this and all other medicines out of the reach of children, never share your medicines with others, and use this medication only for the indications prescribed by your physician.
Every effort has been made to ensure that the information provided by Pharma Synth Formulations Ltd. (‘PSFL’) is accurate, up-to-date, and complete, but no guarantee is made to that effect. PSFL does not endorse drugs, diagnose patients or recommend therapy and is an informational resource designed to assist licensed healthcare practitioners in caring for their patients and/or to serve consumers viewing this service as a supplement to, and not a substitute for, the expertise, skill, knowledge and judgment of healthcare practitioners. PSFL does not assume any responsibility for any aspect of healthcare administered with the aid of information provided. The information contained herein is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or adverse effects. If you have questions about the drugs you are taking, check with your doctor, nurse or pharmacist.