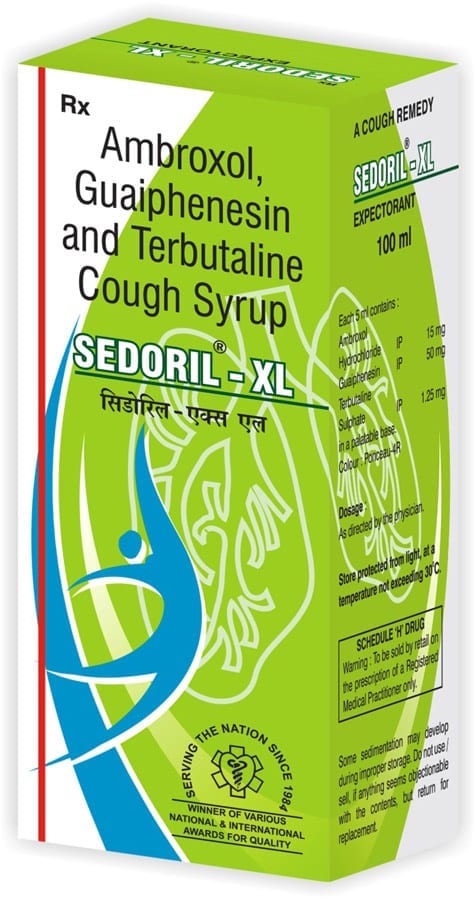
SEDORIL-XL
.:: COMPOSITION ::.
» Bromhexine HCL IP 4mg
» Terbutaline Sulphate IP 1.25 mg
» Guaiphenesin IP 50 mg
in a palatable base
Description
A trusted expectorant for variety of cough.
SEDORIL-XL
Composition:
Each 5 ml of Sedoril-xl contains:
Ambroxol hydrochloride 15 mg
Terbutaline sulphate IP 1.25 mg
Guaiphenesin IP 50 mg
In a flavoured syrup base
Dosage form:
Syrup
ATC classification:
Cough and cold preparations
Description:
Sedoril-XL syrup formula is a combination of a mucolytic agent, an expectorant and a bronchodilator. Ambroxil hydrochloride is the mucolytic agent that reduces the viscosity of bronchial secretions. Guaiphenesin is the expectorant component of Sedoril-XL that loosens congestion in chest and throat making it easier to cough through the mouth. Terbutaline sulphate is the bronchodilator that opens airways and makes breathing easier. This formulation aids in liquefying mucus, soothing irritated bronchial mucosa and makes the cough more productive.
Pharmacological action:
Ambroxol shows mucolytic action by inhibiting the biological signaling by nitric oxide. Nitric oxide enhances the activation of a soluble enzyme called guanylate cyclase. This activation of enzyme is associated with excess mucus secretion and inflammatory disturbances of airways function. In addition, Ambroxol possesses mucokinetic (improvement in mucus transport) and secretolytic (liquifies secretions) properties. It promotes the removal of tenacious secretions in the respiratory tract by improving the mucociliary transport of bronchial secretions and by lowering the phlegm viscosity.
Terbutaline, a selective β2 receptor agonist increases the intracellular cyclic AMP in bronchial muscle cells, which in turn relaxes bronchial smooth muscle and inhibits release of mediators of hypersensitivity, especially from mast cells. Stimulation of adenyl cyclase enzyme by terbutaline through action on β2 receptors enhances conversion of ATP to cyclic AMP. Thus terbutaline causes bronchodilation, increases mucociliary clearance, suppresses edema and has anti-allergic effects.
Guaiphenesin acts by increasing output of sputum and bronchial secretions, reduces the viscosity and adhesiveness of tenacious secretions and acts as an expectorant. Another possible mechanism by which it acts is by increasing the water bonding and volume of the sputum, thereby decreasing its viscosity and leading to an increase in mucokinesis. Increased flow of less viscid secretions promotes ciliary action. Guaiphenesin is effective in both productive and nonproductive coughs.
Pharmacokinetic of Sedoril-XL:
Ambroxol is rapidly absorbed from gastrointestinal tract and it takes 2 hours for ambroxol to reach peak plasma concentration. It undergoes renal excretion. The elimination half-life of ambroxol is biphasic, with an alpha half-life of 1.3 hours and a beta half-life of 8.8 hours.
Terbutaline sulphate is absorbed from gastrointestinal tract and it is subjected to first pass metabolism. It has half life of 3-4 hours. It is excreted via urine as inactive conjugates and also as unchanged drug.
Guaiphenesin is absorbed from gastrointestinal tract and it takes 15mins for guaiphenesin to reach peak plasma concentration. It undergoes oxidation and demethylation. It is excreted via urine.
Indications:
Sedoril-XL syrup is indicated in the treatment of productive cough when associated with bronchospasm in conditions such as:
- Bronchitis and bronchial asthma
- Bronchlectasis
- Chronic obstructive pulmonary disease (COPD)
- Bronchlectasis
- Emphysema
Dosage:
Recommended dosage unless otherwise specified by your Physician:
Children between 2 to 6yrs of age: ½ teaspoon (2.5ml) thrice daily.
Children between 6 to 12yrs of age: 1teaspoon (5ml) thrice daily.
Adults and children 12yrs of age and above: 2 teaspoons (10ml) thrice daily.
Contraindications:
- Sedoril-XL is contraindicated in patients hypersensitive to any of the components of the formulation.
- It is contraindicated in patients with pre-existing ischaemic heart disease or those patients with significant risk factors for ischaemic heart disease.
- It is also contraindicated in patients with gastric ulceration, peptic ulcer of stomach and duodenum and disorder of hepatic function.
Side effects:
Few side effects reported with Sedoril-XL:
Digestive system: nausea, vomiting, dyspepsia, diarrhea, headache and rarely exacerbations of peptic ulcer
Cardiovascular system: palpitations and tachycardia
Central and peripheral nervous system: dizziness, headache, tremor and drowsiness
Respiratory, Mediastinal and Thoracic Disorders: Oral and pharyngeal hypoaesthesia, dry mouth and dry throat.
Allergic reactions are very rare
There have been rare reports of elevations in liver enzymes and of hypersensitivity vasculitis.
Note: In case of overdose immediately get medical help or contact a poison control center right away.
Stop use and immediately consult your doctor if
- Nervousness, dizziness, or sleeplessness occur
- Cough or nasal congestion persists for more than 1 week
- Cough or nasal congestion tends to recur, or is accompanied by a fever, rash or persistent headache.
- New symptoms occur
Warnings and Precautions:
General:
- Doctors or Pharmacist must inquire if the patient is taking sedatives or tranquilizers.
- Doctors or Pharmacist must inquire if the patient has
- Chronic cough such as that occurs with asthma
- Emphysema or chronic bronchitis
- A breathing problem, or persistent or chronic cough such as occurs with smoking, asthma
- Cough accompanied by excessive phlegm (mucus)
- Glaucoma
- Heart disease (high blood pressure)
- Thyroid disease
- Diabetes
- Difficulty in urination due to enlargement of the prostate gland.
- While treating cough as a symptom, it is important to determine and treat the underlying cause, such as a specific infection.
- Caution should be observed while prescribing Sedoril-XL to patients with
- Hypertension, cardiovascular disease including arrhythmias, coronary insufficiency.
- Uncontrolled diabetes mellitus
- Hyperthyroidism
- Patients with history of seizures
- Patients who are unusually responsive to sympathomimetic
- As Sedoril-XL has terbutaline and therefore patients susceptible to hypokalemia should be monitored because transient early falls in serum potassium levels have been reported with β2receptor
- As Sedoril-XL has terbutaline, it should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants, or within 2 weeks of discontinuation of such agents, since the action of terbutaline on the vascular system may be potentiated.
- As bromhexine is a mucolytic, it may disrupt the gastric mucosal barrier. Bromhexine should be used with care in patients with a history of peptic ulceration.
Drug Interactions:
- Terbutaline in Sedoril-XL shows adverse reactions when corticosteroids are concomitantly administered.
- Hypokalemia associated with terbutaline may increase susceptibility to digitalis induced cardiac arrhythmias
- Concomitant administration of aminophylline or xanthenes or diuretics may enhance the risk of hypokalemia
- Since Sedoril-XL has terbutaline sulphate, other sympathomimetic bronchodilators or epinephrine including aerosol bronchodilator of the adrenergic stimulant type should not be used concomitantly as their combined effect on cardiovascular system may be deleterious to the patient.
- β2 blockers not only block the pulmonary effect of terbutaline but may produce severe asthmatic attacks in asthmatic patients. Therefore, patients requiring treatment for both bronchospastic disease and hypertension should be treated with medication other than beta blockers for hypertension.
Pregnancy:
Safety of Sedoril-XL in pregnant and lactating women has not been well studied. Hence this combination should be administered with caution in pregnancy.
Lactation:
Terbutaline is secreted in breast milk, but effect on the infant is unlikely at therapeutic doses. However, this combination should be used with caution in nursing mothers.
References:
https://pubchem.ncbi.nlm.nih.gov/compound/ambroxol#section=Depositor-Supplied-Patent-Identifiers
https://pubchem.ncbi.nlm.nih.gov/compound/Terbutaline_sulfate#section=Drug-Labels-for-Ingredients
https://pubchem.ncbi.nlm.nih.gov/compound/guaifenesin
Disclaimer:
- Information provided above is for reference purpose only and has been compiled for use by healthcare practitioners. Please consult your physician to understand how the product affects you, its dosages, side-effects and further information.
- Remember, keep this and all other medicines out of the reach of children, never share your medicines with others, and use this medication only for the indications prescribed by your physician.
- Every effort has been made to ensure that the information provided by Pharma Synth Formulations Ltd. (‘PSFL’) is accurate, up-to-date, and complete, but no guarantee is made to that effect. PSFL does not endorse drugs, diagnose patients or recommend therapy and is an informational resource designed to assist licensed healthcare practitioners in caring for their patients and/or to serve consumers viewing this service as a supplement to, and not a substitute for, the expertise, skill, knowledge and judgment of healthcare practitioners. PSFL does not assume any responsibility for any aspect of healthcare administered with the aid of information provided. The information contained herein is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or adverse effects. If you have questions about the drugs you are taking, check with your doctor, nurse or pharmacist.